ABC News/WHO
 ABC News/WHOBy ELISSA NUNEZ, ABC News
ABC News/WHOBy ELISSA NUNEZ, ABC News
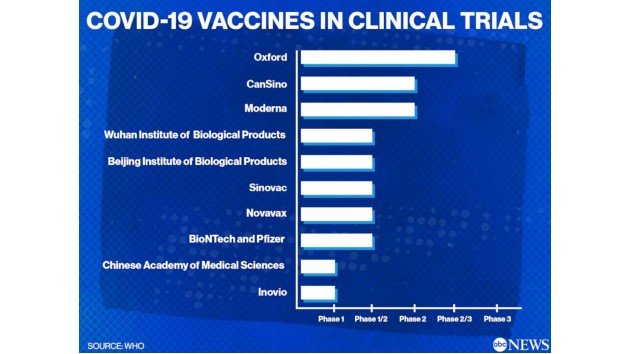
(NEW YORK) — The world’s leading drug companies, universities and governments are racing to develop a vaccine for COVID-19, the disease that has taken more than 400,000 lives globally. Of the 133 candidates being explored, 10 have been approved for human trials, according to the World Health Organization.
Companies and research groups in China, the early epicenter of the coronavirus outbreak, are testing five of those vaccines in human trials.
Meanwhile, U.S.-based companies are involved in the development of four additional vaccines, including one that has National Institute of Allergy and Infectious Diseases Director Anthony Fauci “cautiously optimistic.” The Trump administration established a federal program to make 300 million doses of a successful vaccine available to Americans by January 2021.
Some scientists are testing tried-and-true methods, while others are embracing new technologies like DNA- and RNA-based platforms. The goal is to create a safe, effective vaccine that is easy to replicate — at record speed.
Governments have provided billions in funding to researchers who are accelerating the traditional stepwise approach that includes phases 1, 2 and 3. And many entrants have already partnered with manufacturing companies to start scaling up production before they even know if their vaccine will work.
These accelerated efforts to thwart the pandemic have some officials, including Dr. Fauci, hopeful that a vaccine will be ready by year’s end. But if the vaccines currently in human trials fail — which is possible — that timeline will be extended.
Below are the companies leading the global race for a coronavirus vaccine. There are no approved vaccines or treatments for COVID-19 to date, and we still need more data to know if any of these vaccines will prove safe and effective.
AZD1222, The University of Oxford and AstraZeneca (Phase 2/3)
Perhaps one of the fastest-moving projects is Oxford’s, which is enrolling 10,260 people across the U.K. to begin phase 2/3 human trials. Their AZD1222 vaccine, formerly known as ChAdOx1 nCoV-19, uses a weakened version of a virus derived from chimpanzees. Oxford’s partner, AstraZeneca, has already promised enough manufacturing capacity to produce at least two billion doses of AZD1222.
mRNA-1273, Moderna Therapeutics and the National Institute of Allergy and Infectious Diseases (NIAID) (Phase 2)
This vaccine uses a new type of technology called messenger RNA, which produces genetic instructions that direct cells to make proteins that prevent disease. Early data from Moderna’s first clinical trial show the mRNA vaccine triggered an immune response similar to patients who have recovered from COVID-19. The Massachusetts-based company is quickly moving on to larger human trials as it aims to complete its vaccine trials by year’s end.
Ad5-nCoV, CanSino Biological Inc. and the Beijing Institute of Biotechnology (Phase 2)
CanSino Biologics, a Chinese vaccine company, is creating a viral vector vaccine that combines a weakened virus called adenovirus type 5 with genetic material from SARS-CoV-2. Early results from CanSino’s phase 1 study, which tests for safety, showed the vaccine produced an immune response against the virus and participants tolerated it well. CanSino is collaborating with Canada’s National Research Council to begin research in Canada.
BNT162, Pfizer, Fosun Pharma and BioNTech (Phase 1/2)
Pfizer and BioNTech started dosing the first participants with their vaccine candidate in Berlin in late April. Soon after, the companies began testing four versions of its mRNA-based vaccine at New York University, the University of Maryland, Cincinnati Children’s Hospital Medical Center and the University of Rochester, in May. The companies will narrow down the candidates by safety and how well they prevent infection in patients. Pfizer CEO Albert Bourla said the vaccine could be ready by the end of October.
NVX-CoV2373, Novavax (Phase 1/2)
The Department of Defense recently awarded Novavax $60 million for the manufacture of this protein-based vaccine, which uses patented nanoparticle technology to enhance immune response. In animal studies, it stimulated high levels of antibodies that neutralized the virus. Phase 1 trials are taking place in Herston and Melbourne, Australia. Novavax expects preliminary results in July and, if successful, the company will move onto larger clinical trials in multiple countries.
CoronaVac, Sinovac Biotech (Phase 1/2)
CoronaVac is an inactivated vaccine being tested in China. This type of vaccine uses a killed version of the germ that causes the disease to stimulate an immune response, and has been used to prevent hepatitis A, polio, rabies and the flu. Sinovac published a study in May that showed its vaccine protected monkeys against infection. The company is currently testing 144 people in phase 2 trials.
Unnamed vaccines, Sinopharm (Phase 1/2)
Sinopharm, a state-run Chinese pharmaceutical company, is developing two inactivated vaccines at their subsidiaries, the Wuhan and Beijing Institutes of Biological Products. Both have entered Phase 1/2 testing, according to the WHO.
INO-4800, Inovio Pharmaceuticals (Phase 1)
INO-4800 is a DNA-based vaccine targeting the spike surface protein of SARS-CoV-2 virus that causes COVID-19. Researchers administer the vaccine by skin injection, then follow with Inovio’s CELLECTRA smart device. This delivers DNA to cells using a pulse of electricity that temporarily opens pores in the cell membranes. Early data from preclinical studies shows the vaccine produced a strong immune response in mice and guinea pigs. Phase 1 study is taking place at Central Kentucky Research Associates, the Center for Pharmaceutical Research in Kansas City, Missouri, and the University of Pennsylvania.
Unnamed vaccine, Institute of Medical Biology, Chinese Academy of Medical Sciences (Phase 1)
The Institute of Medical Biology at the Chinese Academy of Medical Sciences (IMBCAMS) has previously developed the world’s first inactivated vaccines for polio and hand-foot-and-mouth disease, according to the company website. This inactivated vaccine is in phase 1 trials for safety, according to the WHO.
Copyright © 2020, ABC Audio. All rights reserved.